Submitted by Sammy Snyder on
The uChek urine analyzer app for iOS devices has come under fire from the U.S. Food and Drug Administration. The FDA has sent a letter to BioSense Technologies asking why their app hasn't been cleared with the agency. The uCheck app allows users to check their glucose and other analyte properties by using the iPhone's camera to read urine strips.
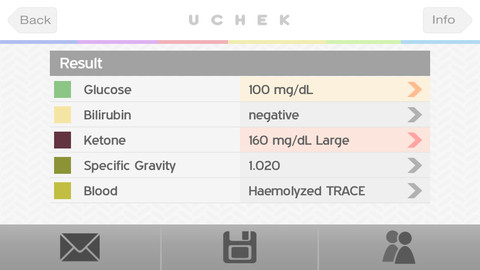
This is the first time a medical app has drawn the attention of the FDA, according to Bloomberg. The FDA says uCheck requires additional approval because it reads test strips made by Siemens AG (SIE) and Bayer AG (BAYN). The agency also believes there needs to be stricter rules for apps that replace existing medical devices, or that directly diagnose medical conditions.
“We intend to finalize the guidance this year,” Synim Rivers, an agency spokeswoman, said yesterday in an e-mail. “The FDA has proposed a regulatory approach that limits its immediate oversight to a specific, small subset of mobile medical applications that are medical devices and present the greatest risk to patient safety if they don’t work as intended.”
The uCheck app only works with the BioSense Technologies' automated urinalysis kit, which can be purchased for $40. The app itself is free to download from the Apple App Store. It's compatible with the iPhone, iPod touch, and iPad, and requires iOS 5.1 or later.